Acidification
Acidi-what?
Acidification is the name given to the ongoing derease in the pH-value of the Earth's oceans.
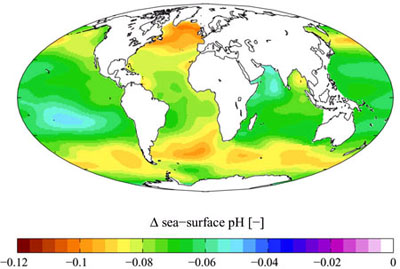
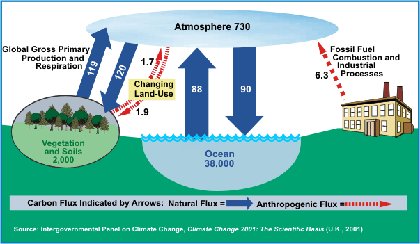
The oceans absorb carbon dioxide (CO2) from the atmosphere, thus causing chemical changes by making them more acidic (that is, decreasing the pH of the oceans). In the past 200 years, the oceans have absorbed approximately half of the CO2 produced by fossil fuel burning and cement production.
How does ocean acidification affect marine life?
While this absorption provides a great service to humans by significantly reducing the amount of greenhouse gases in the atmosphere and decreasing the effects of global warming, it induces changes in the ocean chemistry. These changes in chemistry affect marine life and particularly organisms with calcium carbonate shells, such as corals, mussels, mollusks and small creatures in the early stages of the food chain.
And what has acidification to do with calcium carbonate shells?
Corals, calcareous phytoplankton, mussels, snails, sea urchins and other marine organisms use calcium (Ca) and carbonate (CO3) in seawater to construct their calcium carbonate (CaCO3) shells or skeletons.
As the pH-value decreases, carbonate becomes less available, which makes it more difficult for organisms to secrete CaCO3 to form their skeletal material.

Source: NOAA Coral Reef Watch.
Uncertain future…
Since the oceans have never experienced such a rapid acidification, it is not clear if ecosystems have the ability to adapt to these changes.
Effects of ocean acidification on organisms and ecosystems are still poorly understood. Over the last few years, research has intensified significantly to fill the many knowledge gaps.
