Supplement 1.7: Radiation quantities and radiometry (8/9)
Photosynthetically Active Radiation PAR
(also known as Photosynthetic Photon Flux Density PPFD)
Measurements of radiation, which is essential for photosynthesis in vegetation, are of particular importance in biology. Since every photon absorbed by a plant's photosystem can contribute to photosynthesis, the wave model of light is not very suitable and data in units of W/m² are not relevant. Rather, photosynthesis is a quantum mechanical process and therefore the number of photons in the spectral range used by plants is of interest.
This range extends from 400 nm (dark blue) to 700 nm (dark red) and roughly corresponds to visible light. The radiation can be described as the quantum irradiance of photosynthesis. It is the photosynthetically active radiation (PAR). Since photons are not further differentiated, a term borrowed from chemistry was introduced for photon numbers: the mole, which is referred to here as an Einstein. One Einstein therefore corresponds to 6·1023 Photonen.
Formula symbol:
Unit of measurement: Photons/(area·time),
Approximately 500 μEinstein/(m²s) are required for healthy plant growth.
The energy of a photon is ,
with Planck's constant h=6.626·10-34 J s, the speed of light c=2.998·108 m/s, and the frequency f and wavelength λ of the corresponding electromagnetic wave. The optical power of the photons is determined by their photon energy and their number n per time. For a differential photon number dn with photon energy E in a wavelength interval dλ, the optical power is:
Now photons from 400 to 700 nm are to be counted, whereby their wavelength must not play a role. In the right-hand column of the first page of this supplement, the spectral sensitivity of a detector was introduced as the ratio of the electrical output signal to the incident optical power. It is denoted here by S.

If the spectral sensitivity applies:
the sensitivity in the relevant spectral range increases proportionally to the wavelength, then:
This spectral characteristic can be achieved with a semiconductor photodiode and a combination of optical filters
in front of the sensor surface. Such PAR sensors are widely available.
Alternatively, a spectrometer can be used if its spectral sensitivity is adjusted with a correction function in the software so that a proportional increase with wavelength is achieved in the relevant spectral range.
One disadvantage of the process described above is that photons are always weighted equally, regardless of their energy. This should be the case, but the probability of absorption by plant photosystems is not independent of photon energy or wavelength. Chlorophyll a absorbs in the blue and red ranges, but only to a small extent in the green range. Plant photosystems consist of other absorbing pigments such as chlorophyll b, carotene and xanthophyll, which together form the so-called light-harvesting complexes (LHC). It would make sense to take this into account when counting photons.
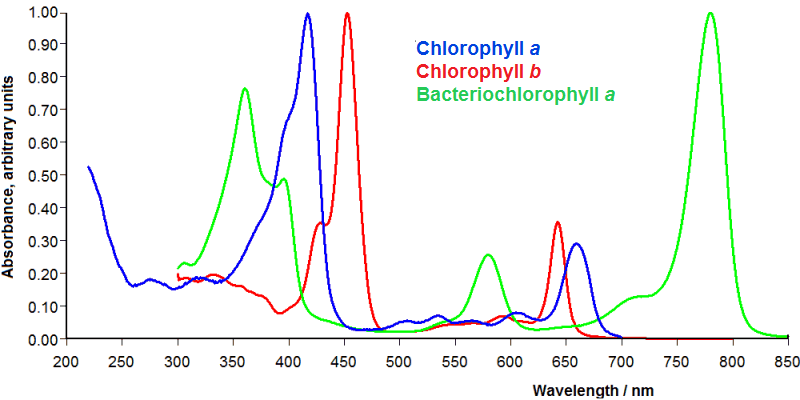
Source: PhotochemCAD
A spectrometer offers the advantage of weighting light according to these properties. This method leads to another variable known as Photosynthetically Usable Radiation PUR or Photosynthetic Photon Flux Density PPFD). This data is also expressed in the unit Einstein/(m²s).
