3. Climate and climate change
The solar radiation on the Earth's surface
In the atmosphere on a clear sunny day with the sun high in the sky, about 40% of the radiation is lost through absorption and scattering. In other words, under the most favourable conditions, around 60% of solar radiation reaches the earth's surface; this has a value of about 1000 W/m².
Scattering is caused by all atoms and molecules in the atmosphere. It occurs on the gas molecules as the so-called Rayleigh scattering and is what creates the blue colour of the sky, and also makes the Earth appear blue from space.
Nitrogen in the air has no effect in absorption; oxygen plays only a very minor role. It is almost exclusively trace gases that absorb the light. The visible light spectrum is also hardly influenced by the absorption of these trace gases. The following illustration shows that the wavelengths from 0.4 to 0.75 µm (or: 400 to 750 nm) make up the atmospheric window of solar radiation in the visible spectral range.
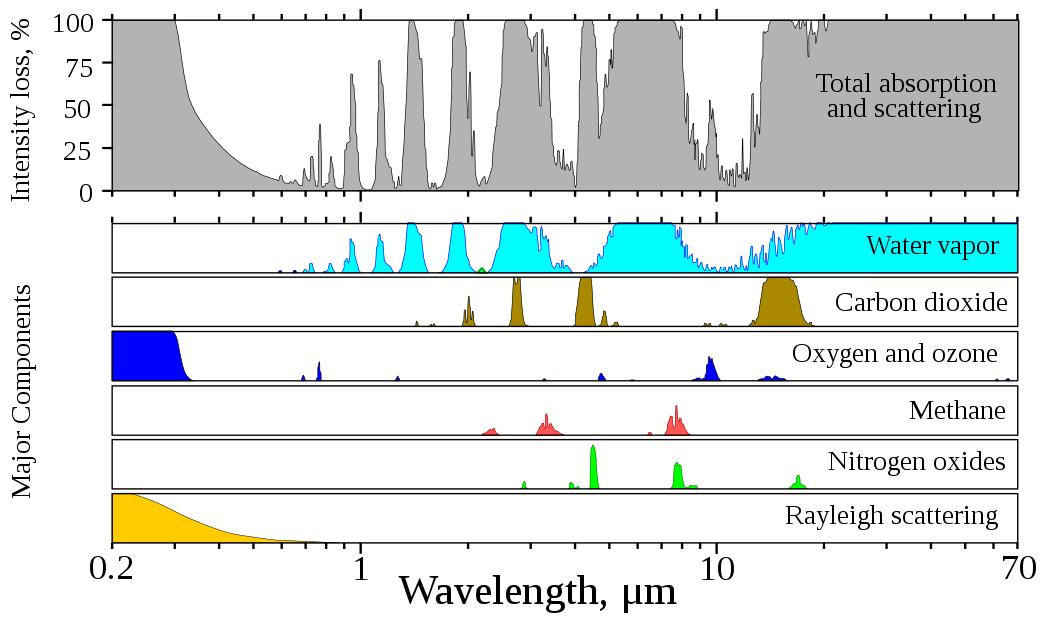
Above: Percentage of radiation loss when solar radiation passes through the atmosphere, plotted against the logarithm of the wavelength. The sun is at its zenith, the air is clear and free of clouds. Bottom: Contributions to absorption from the most important trace gases as well as air molecules for Rayleigh scattering.
Source: Wikimedia Commons, edited.
The most important trace gases are:
- the ozone (O3) in the stratosphere. It protects us from the harmful ultraviolet radiation from the sun. However, if it reaches ground level through pollutants such as car exhausts, it damages our lungs.
- carbon dioxide and nitrogen oxide produced when coal, oil and gas are burned,
- methane that is produced in rice cultivation, as a result of cattle breeding, and released from melting permafrost,
- water vapor, which evaporates in increasing amounts into the atmosphere with rising temperatures.
In addition, there are particles in the air such as dust, soot, haze, fog and clouds. Their appearance and behaviour is very variable and creates our ever changing weather. As an example the following diagram shows that even on a monthly average, there are vastly different values of solar exposure.
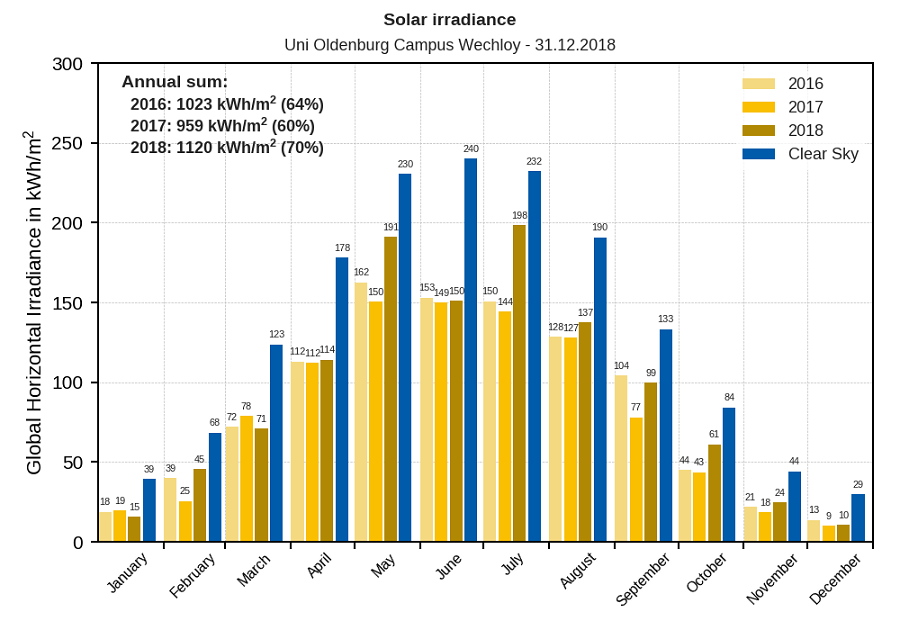
Source: Energy Meteorology, University of Oldenburg, Germany.
