4. الامتصاص والتشتت
Absorption photometry (3/6)
The disadvantages of a single beam photometer are inherent in the time-consuming measurements of the reference and the actual sample for only one analysis. The content of the cuvette has to be exchanged or even the whole reference cuvette has to be replaced by the sample cuvette. This procedure easily leads to inaccuracy of the optical alignment which is not always noticeable. Therefore the risk of measuring inaccurate data results.
Collecting data for both cuvettes at the same time would be by far more convenient. This requires an alignment with two optical paths.
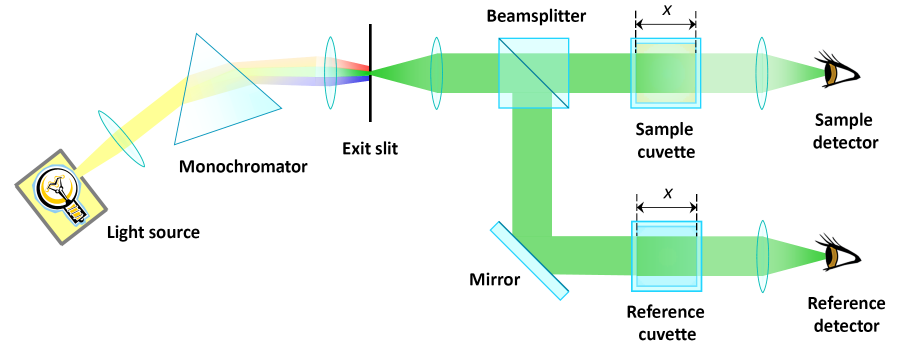
The double beam photometer
The light from the monochromator is splitted by a beam splitter into two seperate beams. Now we have two beams at our disposal for the two cuvettes. The transmitted light is measured by two photodetectors. The solvent can rest in the reference cuvette and will be examined in every analysis as well as the differing samples.
Instead of a beam splitter, spinning glass panels can be used. They are partially mirrored so that the monochromatic light is alternately lead through to the sample cuvette and reflected to pass the reference cuvette.
It could be objected that the two setups are no longer identical. This was a prior condition for data analysis as shown on the previous page. In this particular case, one finds two different lenses and light detectors.
The objection is justifiable. However, the expected differences in the distances can be solved throughout a trick. Both cuvettes will be filled with the same reference liquid (mostly purified water) at the beginning and the signals of the detectors are measured. This is carried out for all the wavelengths of which the absorption coefficients shall be analysed. The observed deviations of the signals are consequently caused by the different ray paths and sensitivities of the detectors.
The photometer's software then deduces factors from those deviations which help to correct the signals later-on for the purpose of making the double beams numerically equal to each other. This electronical signal adjustment is mostly called 'autozero'.
A subsequent measurement with the reference liquid in both cuvettes ought deliver a value around zero for the extinction. That is a test for the correct optical and electronic adjustment of both light ways. Henceforth the photometer is prepared for the analysis of the absorbing features of samples.
On the next page is shown a modern double beam photometer, as it is available in all chemical and physical laboratories.