Worksheet 4.1: How to construct an absorption photometer on your own
In the section about absorption of light, Lambert's law, which shows the decline in the intensity of light on its way through an absorbing medium with an absorption coefficient , has been presented like this:
In experiments on photometry a glass cuvette of the length is filled with a sample and lighted. At the entrance of the cuvette (at ) the intensity equals . The by absorption lowered intensity is measured at the end of the cuvette. It is aim of the measurement to calculate the absorption coefficient from the data of and such as the known length of the cuvette.
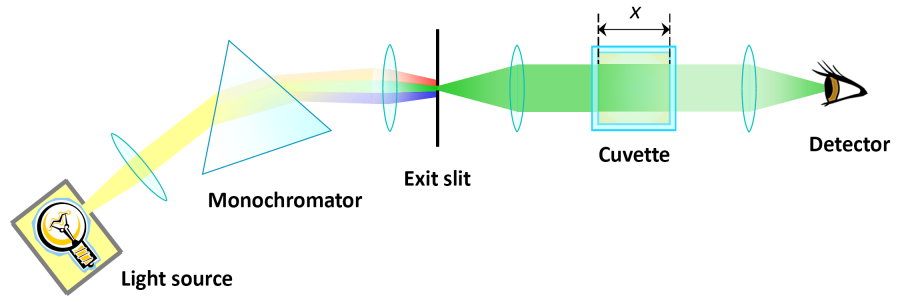
Suggestions for the construction of a photometer
What you need:
- a light source; halogen lamps are most suitable because of their brightness and their broad spectrum which covers the visible range. Their brightness is low in the blue range and it is missing in the ultraviolet. If the blue range is of interest, a cool white emitting LED is the better choice.
- converging lenses are easily accessible at stores for teaching materials or via the internet. A lens with a focal length of about 50 mm will work to focus the light from the halogen lamp on the entrance of the spectograph. You can even try whether your spectacle glasses work. A pocket lamp of which the light can be well focussed is suitable as well.
- a monochromator or a spectrograph for the separation of the light into narrow spectral (monochromatic) parts. This is a relatively expensive component. Perhaps it can be found in the collection of physics experiments at school or otherwise it can be borrowed from a scientific research institute. Constructing a monochromator yourself would be an other big project.
- alternatively, single-colour light sources can be used. These would replace a monochromator. As LEDs are available in all colours, they suit this purpose perfectly. Their position in the schematic drawing is at the exit slit, which is no more needed in that case such as all components located further left. Its light is parallelised as good as possible by a converging lens. LEDs with a clear plastic package are of advantage in comparison to those with an opaque plastic package.
- a cuvette for the presentation of liquid samples. A vase with a rectangular cross section will work as long as the glass' surface is plane.
Stores for laboratory equipment often sell low-cost plastic cuvettes, and Pasteur pipettes.
Those enable us to fill and empty the cuvette without detaching it from the test set-up. That way the optical alignment remains intact.
Especially the left cuvette suits well for the purpose of photometry because the two polished sides face the two opaque ones. The opaque surfaces reduce reflections of the transmitted light. The polished cuvette on the right might be used for fluorescence analyses as well, which are executed in a 90° arrangement of the exciting and detection light paths. Some kinds of plastic can be damaged by alcohol so that they can only be used with aqueous solutions.
 Cuvettes for photometry in chemical laboratories in most cases come in inner dimensions of 1 cm × 1 cm × 5 cm. They are available made from glass and (as shown above) made from plastic.
Cuvettes for photometry in chemical laboratories in most cases come in inner dimensions of 1 cm × 1 cm × 5 cm. They are available made from glass and (as shown above) made from plastic. - a photodetector. Very reasonable and sensitive at the same time is a silicon photodiode, which generates a photo current proportional to the incoming light intensity. The photo current is indicated by an ammeter of which the effective range has to be sensitive to 0.1 μA or better.
For more suggestions concerning the construction of a photometer and the avoidance of measurement errors have a look at the additional hints. The collection of data and its analysis is discussed in the section Analysing photometer data.
