Supplement 2.5: Weathering of Oil on the Water Surface (4/4)
- Chemical and Biological Processes -
7. Photo-Oxidation (Chemical process)
Hydrocarbons can react with oxygen, which may either lead to the formation of soluble products or persistent tars. Oxidation is promoted by sunlight; it's rate is directly linked to light intensity and slick thickness.
Oxidation of hydrocarbons is a very slow process; it has little influence on the short term evolution of an oil spill. Even under intense sunlight, thin oil films break down only slowly, and usually less than 0.1% per day. Thick layers of very viscous oils or water-in-oil emulsions tend to oxidise to persistent residues rather than degrade, as higher molecular weight compounds are formed that create a protective surface layer as is the case with tarballs (ITOPF 2002).
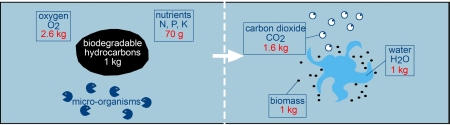
8. Biodegradation (Biological process)
Sea water contains a range of marine micro-organisms capable of metabolising oil compounds. They include bacteria, moulds, yeasts, fungi, unicellular algae and protozoa which can utilise oil as a source of carbon and energy.
Such organisms are distributed widely throughout the world's oceans although they tend to be more abundant in chronically polluted coastal waters, such as those with regular vessel traffic or which receive industrial discharges and untreated sewage. The main factors affecting the rate and extent of biodegradation are the characteristics of the oil, the availability of oxygen and nutrients (principally compounds of nitrogen and phosphorus) and temperature.
Because the micro-organisms live in the water, from which they obtain oxygen and essential nutrients, biodegradation can only take place at an oil/water interface. At sea, the creation of oil droplets, either through natural or chemical dispersion, increases the interfacial area available for biological activity and so may enhance degradation.
In contrast, oil stranded in thick layers on shorelines or above the high water mark will have a limited surface area and will be subject to drier conditions which will render degradation extremely slow, resulting in the oil persisting for many years. Similarly, once oils become incorporated into sediments on the shoreline or sea bed, degradation is very much reduced or may stop due to a lack of oxygen and/or nutrients. The variety of factors influencing biodegradation makes it difficult to predict the rate at which oil may be removed. Although biodegradation is clearly not able to remove bulk oil accumulations, it is one of the main mechanisms by which dispersed oil or the final traces of a spill on shorelines are eventually removed.