Supplement 2.3: Emulsions
Why do oil and water not mix?
Some emulsions are quite stable and will take a long time to separate. For example milk is an emulsion of water and fat but is fairly stable. Other emulsions separate quite quickly, for example a simple salad dressing of oil and vinegar will separate almost immediately. This is because oil is less dense than vinegar which is water based.
The emulsion itself consists of small droplets of one liquid within the body of a second liquid. An emulsion containing droplets
of oil in water is called an oil-in-water emulsion and the oil is called the dispersed phase while the water
is called the continuous phase.
The other case, i.e. water being the dispersed phase and oil being the continuous phase, is a water-in-oil emulsion.
This process is also refered to as reverse emulsification.
The Physics of Emulsification
All surfaces have a surface energy which is responsible for phenomena such as surface tension. If you place a drop of oil into a glass of water a new surface at the interface between the oil and water is created and this surface will have an energy
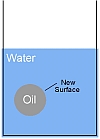
This energy must be provided from somewhere. Thus to create an emulsion from oil and water you must supply energy! For example you must shake a salad dressing to create an emulsion from the oil and vinegar, if you do not shake it the two liquids stay in separate layers.
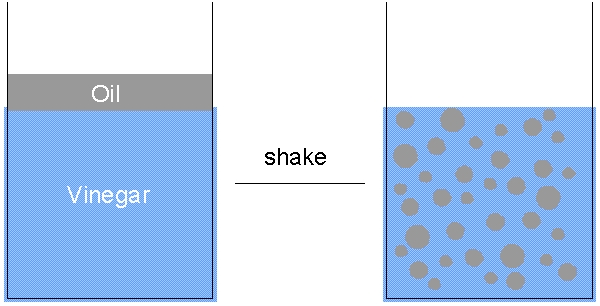
All systems try to reduce their energy to a minimum. Thus the drop-of-oil-in-water system wants to reduce the area of the oil-water interface. Less surface area equals less surface energy. For a given volume of oil the minimum surface area possible is obtained by forming a sphere; therefore oil drops in water and bubbles of gas in a liquid are always spherical.
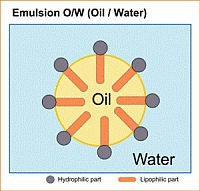
Oil-in-water emulsions (o/w)
With o/w-emulsions the water phase is continuous, whereas the oil phase is distributed in the water in the form of small droplets. An o/w emulsion contains at least 26% of water.
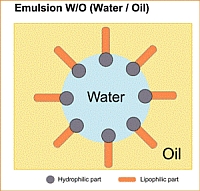
Water-in-oil emulsion (w/o)
With w/o emulsions the conditions are the other way round: the oil phase is continuous and the water forms droplets in the oil. The water content in w/o emulsions is no more than 74%.
However, the type of emulsion is not determined by the water content but by the type of emulsifier!
Emulsifiers
Oil-in-water emulsions form when emulsifiers with hydrophilic (water-loving) groups dominate whereas hydrophobic (oil-loving) groups lead to the formation of water-in-oil emulsions. An emulsifiers positions itself at the oil-water or air-water interface and, by reducing the surface tension, has a stabilising effect on the emulsion.
Question: Can you think of oil-in-water and water-in-oil emulsions from your everyday life ?
